Kareena Dhillon
Contributing Writer
UC Santa Barbara (UCSB) professor M. Scott Shell, Department Vice Chair of chemical engineering, runs the “Shell Lab” on campus to help graduate and undergraduate students conduct research on water solutions, peptide structure, advanced simulation techniques, and many more. One of their latest research projects focuses on purifying water here on campus.
In an age where one in three people do not have access to safe drinking water, according to the World Health Organization, Shell’s research is crucial to helping the global population. In an interview with The Bottom Line, Shell explained this project in detail.
Shell and his colleagues at UCSB and several faculty members at the University of Texas at Austin are working towards manipulating polymeric membranes to filter water. Polymeric membranes are used in water purification systems because they are able to separate small particles that are present in water from the water itself. Shell’s research team is studying small molecule solutes’ ability to improve the water purification process.
Shell and his colleagues at UCSB and several faculty members at the University of Texas at Austin are working towards manipulating polymeric membranes to filter water. Polymeric membranes are used in water purification systems because they are able to separate small particles that are present in water from the water itself. Shell’s research team is studying small molecule solutes’ ability to improve the water purification process.

“Only water fits through this polymeric membrane, it’s got very, very tiny, you can think about importers, but they’re so tiny I would probably call them pores. And it requires you to pressurize the stream of water and the saltwater that comes through … is your clean water.”
This purification method works because only water is able to fit through the membrane. In addition, “the reason that it’s such a nice technology is that the energy use is really low compared to other ways of purifying water,” Shell explained.
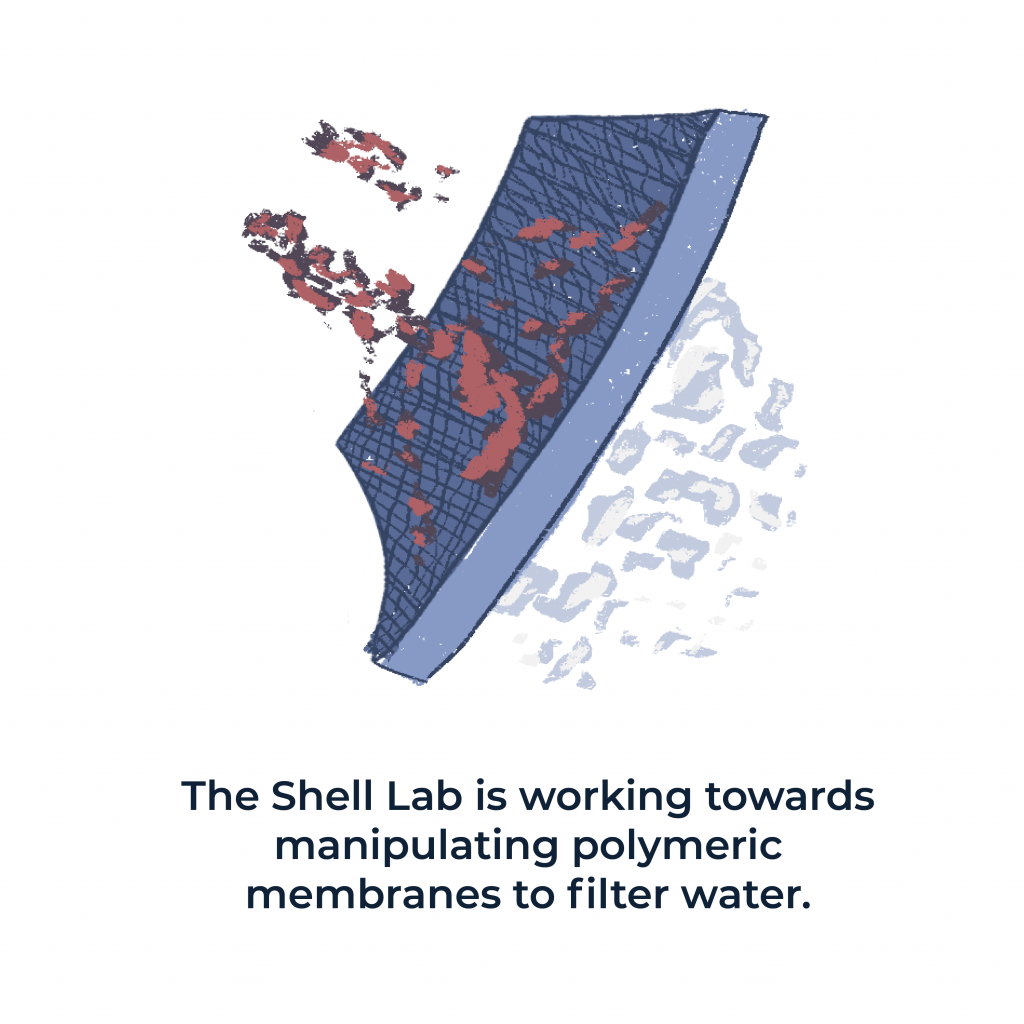
Water purification membranes are limited by the inherent design and convention of membrane materials, considering how easily they can be clogged and deteriorated. However, recent advances can control the structure and chemical functions of polymer films, or barriers to protect the water from impurities, to potentially lead to new classes of membranes aiding in the purification of water and utilize the ability to capture resources from the unpurified water.
The polymeric membranes used to filter out saltwater can be incredibly thin on a molecular level, yet Shell and his colleagues have been able to study the membranes themself and even begin to “re-engineer these membrane technologies” to address key issues with water purification.
For example, some groups in the Shell Lab are working to capture lithium, using the process of selectivity, considering it is a “very valuable component used in batteries.” Selectivity is the process that allows “one type of molecule to go through in a membrane versus another.” To put it simply, “you’re basically coating the surface of your membrane with different functional groups so you can engineer what goes through and what does not.” In case, functional groups are molecules or groups of molecules that dictate whether or not certain molecules present in the water go through the membrane.
For example, some groups in the Shell Lab are working to capture lithium, using the process of selectivity, considering it is a “very valuable component used in batteries.” Selectivity is the process that allows “one type of molecule to go through in a membrane versus another.” To put it simply, “you’re basically coating the surface of your membrane with different functional groups so you can engineer what goes through and what does not.” In case, functional groups are molecules or groups of molecules that dictate whether or not certain molecules present in the water go through the membrane.

The team worked to determine how water molecules near the surface are structured and how they move. They wanted to know “how a surface impacts the behavior of water.” Through their research they found that “hydrophilic molecules liked to stick to hydrophobic surfaces,” and vice versa. This was a breakthrough discovery at Shell’s lab because typically hydrophilic molecules are characterized as molecules that are attracted to water, while hydrophobic surfaces are characterized as surfaces that are resistant to water.
This is because “different types of surfaces make water respond differently,” therefore surface fluctuations influence the stickiness of small molecules. This exciting discovery means researchers can now focus on the response of water molecules to different surfaces instead of using conventional methods.
Shell elaborated that water purification can often be influenced by certain molecules in desalination plants. In a process commonly known as fouling, “gunky stuff sticks to your membrane surface and clogs it up so that your performance goes down over time.”
Fouling is a constant obstacle for engineers trying to design efficient membranes. Modification of the membrane surface is one solution to avoiding membrane fouling, as it helps to maintain high levels of water productivity.

Not to mention, membranes are not one-size-fits-all, especially when it comes to heavily polluted waters and oil spills. Instead of creating new membranes, which is a very costly and time-consuming process that is not yet within reach for the scientific community, Shell and his colleagues are trying to re-engineer existing membranes.
Looking to the future, surfaces composed of different types of molecular chemistries may be the key to creating durable membranes. Shell and his team have concluded that “different spatial arrangements” of a surface makes it possible to increase or decrease its surface affinity. These findings can significantly improve next-generation membrane systems for sustainable water treatment.
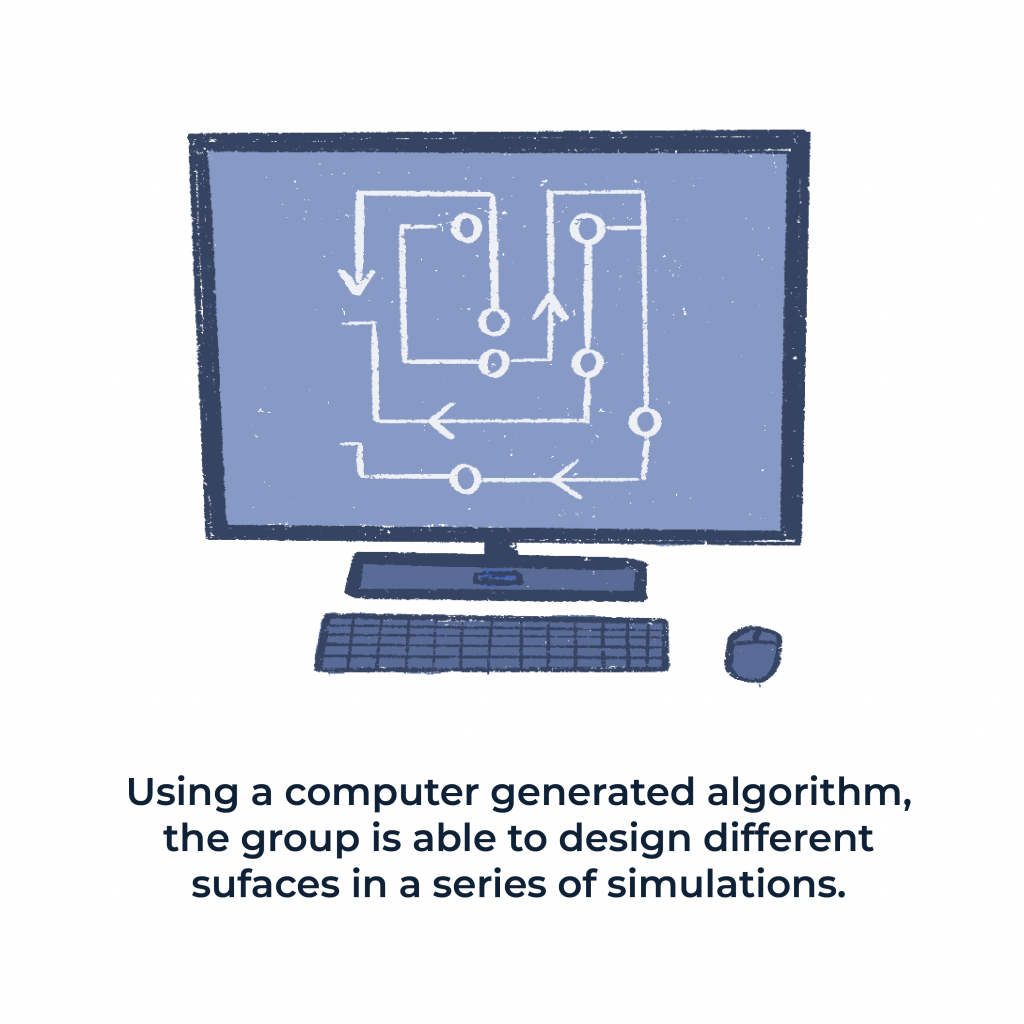
Using a computer generated algorithm, created by Shell’s student Jacob Monroe, the group is able to design different surfaces in a series of simulations. Through the series of simulations the team demonstrates how simulations can implement practical materials design for efficient water treatment.
In a larger context, these simulations can revolutionize technologies involving water-mediated, solute-surface interactions. Shell and his students have combined optimization algorithms and machine-learning tools to make remarkable discoveries here on campus.
This will allow them to test theories and maximize water filtration processes by gaining “the ability to design complicated surfaces that have patterns of different kinds of chemical groups to adjust the function of each surface.” Shell’s lab findings can be found in more depth at “Proceedings of National Academy of Sciences of the United States of America.”
Their findings can also target heavily contaminated samples, such as water found in oil fields. Their next step will be to examine increasingly more complex and realistic surfaces to mimic the function of membrane in a water treatment.
With growing urbanization and climate change, which causes water scarcity all around the world, Shell’s research is crucial to societal progress and adaptation. With his team working to address the obstacles of water purification, more people can have access to clean water in the future.
Illustrations by Esther Liu